Chemistry plays an important role in everyday life, thus improving the quality of life and livelihood. Chemistry is one of the most important subjects students learn in their school life. Learning chemistry is mandatory as it falls out to be a crucial subject in higher classes. The important questions of ICSE Class 8 Chemistry will help students to refresh all the important topics learnt in class 8. All these important questions are prepared by our subject expert by referring to the Chemistry textbook.
Students of Class 8 ICSE should practise these important questions which cover all the essential topics from each chapter. By having a thorough knowledge of the important questions students can attempt the final question paper confidently. It will help them in fetching wonderful marks. These important questions of Chemistry are compiled by referring to the ICSE Class 8 Chemistry syllabus as prescribed by the Board.
Download ICSE Class 8 Chemistry Important Questions PDF
The ICSE Class 8 Chemistry important questions mentioned below will help students to analyze their preparation so that they can improve it before the final exam. These ICSE Class 8 Important Questions for Chemistry will give you a quick recap of the entire Chemistry syllabus.
-
- Which of the following pictures represent the Bohr’s model of an atom?
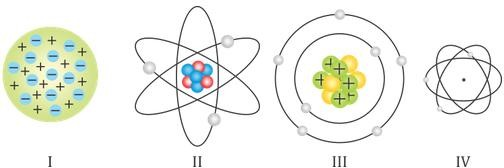
-
- I
- II
- III
- IV
- Sodium forms sodium cation by the loss of
- One electron
- Two electrons
- Three electrons
- Four electrons
- Hydrogen frees metals from their
- Sulphates
- Oxides
- Nitrates
- Chlorides
- State the electronic configuration of the following atoms:
- Atom ‘A’ (Atomic number = 12)
- Atom ‘B’ (Atomic number = 19)
- Atom ‘C’ (Atomic number = 7)
- Atom ‘D’ (Atomic number = 26)
- Atom ‘E’ (Atomic number = 10)
- Fill in the blanks and rewrite the sentences:
- When a few drops of phenolphthalein are added to sodium hydroxide, the solution turns_____.
- Separation of components of air such as liquid nitrogen and liquid oxygen is possible by_____.
- The three states of matter are classified on the basis of differences in certain _____ properties.
- A mixture which has different composition and properties in different parts of their mass is called a_____mixture.
- _____ is used in the laboratory for the preparation of hydrogen using dilute hydrochloric acid.
- State whether the following statements are true or false. Rewrite the false statement.
- A compound is a pure substance composed only of one kind of element combined chemically in a fixed proportion by mass.
- A chemical reaction in which two or more substances combine to form a single product is called a combination reaction or synthesis.
- In the formation of ammonia from hydrogen and nitrogen iron is used as a positive catalyst.
- Magnesium has atomic number 12 and atomic mass number 24.
- The insoluble solid settles down in a beaker is called sediment.
- Name the following:
- Conversion of a solid to a liquid on heating
- Conversion of a liquid to a vapour (or gas)
- Conversion of vapour (or gas) to a liquid
- Conversion of a liquid to a solid
- The outermost shell or orbit of an atom.
- Give distinguishing explanation between alpha rays, beta rays and gamma rays.
- What is a metal reactivity series? What are its important features?
- Define the following:
- Lamp black
- Sugar charcoal
- Chemical equation
- Orbit
- Bone charcoal
-
- Match the name of the radical given in Column A with its formula given in Column B.
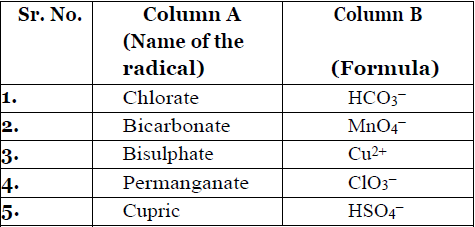
- Write the formula of the given compounds:
- Acetic acid
- Sodium hydroxide
- Sulphuric acid
- Hydrochloric acid
- Ammonium hydroxide
- Write the electronic configuration of the following elements:
- Sodium
- Chlorine
- Hydrogen
- Nitrogen
- Oxygen
- Differentiate between compound and mixture.
- What is atomicity? Give one example each of monoatomic and diatomic molecule.
- Give the chemical equations for a reaction of potassium with
- Oxygen
- Water
- Write the main features of Rutherford’s atomic model.
- How can you prove that hydrogen burns in air to produce water?
- Differentiate between oxidation reaction and reduction reaction.
- What are protons, neutrons and electrons?
Advantages of ICSE Class 8 Chemistry Important Questions
- It eases the revision part and cut it into a short duration of time.
- It covers all the necessary topics from each chapter.
- Solving these important questions will gain confidence among the students.
- They will get an idea to some extent about the types of question to expect from each chapter.


Comments