1
Question
Explain the structure of carbon dioxide.
Explain the structure of carbon dioxide.
Open in App
Solution
Structure of Carbon Dioxide
- The carbon dioxide molecule has two double bonds between Carbon and Oxygen atoms.
- A double bond consists of a sigma and a pi bond which means in total contains sigma and pi bonds.
- The bond angle between Carbon and two Oxygen is 180° as it is linear in structure.
- The hybridization of in is and is hybridized.
- With the electronegativity difference in and, the bonds formed in are polar.
- But because of two Oxygen molecules on each side of Carbon, the dipole moment gets canceled, and the compound has no final dipole moment.

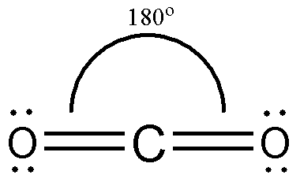
Structure of Carbon Dioxide
- The carbon dioxide molecule has two double bonds between Carbon and Oxygen atoms.
- A double bond consists of a sigma and a pi bond which means in total contains sigma and pi bonds.
- The bond angle between Carbon and two Oxygen is 180° as it is linear in structure.
- The hybridization of in is and is hybridized.
- With the electronegativity difference in and, the bonds formed in are polar.
- But because of two Oxygen molecules on each side of Carbon, the dipole moment gets canceled, and the compound has no final dipole moment.


Suggest Corrections
0
Join BYJU'S Learning Program
Join BYJU'S Learning Program
