JEE Advanced Paper 2 | 2018
Q. Aniline reacts with mixed acid (conc. HNO3 and conc.H2SO4) at 288 K to given P(51%), Q(47%) and R(2%). The major product(s) the following reaction sequence is/are:


View Solution
Q. Galena (an ore) is partially oxidized by passing air through it at high temperature. After some time, the passage of air is stopped, but the heating is continued in a closed furnace such that the contents undergo self-reduction. The weight (in kg) of Pb produced per kg of O2 consumed is________.
[Atomic weight in g mol−1:O=16, S=32, Pb=207]
[Atomic weight in g mol−1:O=16, S=32, Pb=207]
View Solution
Q. In the following reaction sequence, the amount of D (in g) formed from 10 moles of acetophenone is:
(Atomic weight in g mol−1:H=1, C=12, N=14, O=16, Br=80. The yield (%) corresponding to the product in each step is given in the parenthesis).
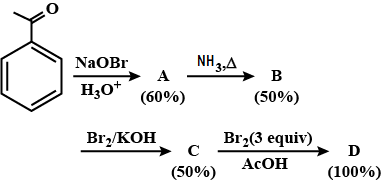
(Atomic weight in g mol−1:H=1, C=12, N=14, O=16, Br=80. The yield (%) corresponding to the product in each step is given in the parenthesis).

View Solution
Q. For a first order reaction A(g)→2B(g)+C(g) at constant volume and 300 K, the total pressure at the beginning (t=0) and at time are P0 and Pt respectively. Initially, only A is present with concentration [A]0, and t1/3 is the time required for the partial pressure of A to reach 1/3rd of its initial value. The correct option(s) is/are:
(Assume that all these gases behave as ideal gases).
(Assume that all these gases behave as ideal gases).
View Solution
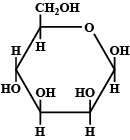
Q. The Fischer presentation of D−glucose is given above.
The correct structure(s) of β−L− glucopyranose is/are:

The correct structure(s) of β−L− glucopyranose is/are:

View Solution
Q. The surface of copper gets tarnished by the formation of copper oxide. N2 gets was passed to prevent the oxide formation during heating of copper at 1250 K. However, the N2 gas contains 1 mole % of water vapour as impurity. The water vapour oxidises copper as per the reaction given below:
2Cu(s)+H2O(g)→Cu2O(s)+H2(g)
ρH2 is the minimum partial pressure of H2 (in bar) needed to prevent the oxidation at 1250 K. The value of ln(ρH2) is _________.
2Cu(s)+H2O(g)→Cu2O(s)+H2(g)
ρH2 is the minimum partial pressure of H2 (in bar) needed to prevent the oxidation at 1250 K. The value of ln(ρH2) is _________.
Given:
Total pressure =1 bar
R (universal gas constant) =8 JK−1mol−1
ln(10)=2.3
Cu(s) and Cu2O(s) are mutually immiscible
At 1250 K:
At 1250 K:
2Cu(s)+1/2O2(g)→Cu2O(s); ΔG∘=−78, 000 J mol−1 H2(g)+1/2O2(g)→H2O(g); ΔG∘=−1, 78, 000 J mol−1
[Upto one decimal point and take modulus of the answer]
View Solution
Q. The correct option(s) regarding the complex [Co(en)(NH3)3(H2O)]3+ (en=H2NCH2CH2NH2) is/are:
- It is paramagnetic.
- It will have three geometrical isomers if bidentate 'en' is replaced by two cyanide ligands.
- It has two geometrical isomers.
- It absorbs light at a longer wavelength as compared to [Co(en)(NH3)4]3+.
View Solution
Q.
For the given compound X, the total number of optically active stereoisomers is __________.

View Solution
Q. The correct option(s) to distinguish nitrate salts of Mn2+ and Cu2+ taken separately is/are:
- Mn2+ shows the characteristic green colour in the flame test.
- Only Cu2+ shows the formation of precipitate by passing H2S in acidic medium.
- Only Mn2+ shows the formation of precipitate by passing H2S in faintly basic medium.
- Cu2+/Cu has higher reduction potential than Mn2+/Mn (measured under similar conditions).
View Solution
Q. For a reaction, A⇌P, the plots of [A] and [P] with time at temperature T1 and T2 are given below.
If T2>T1, the correct statement(s) is/are:
(Assume △Hθ and △Sθ are independent of temperature and ratio of lnK at T1 to lnK at T2 is greater than T2T1. Here H, S, G and K are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)

If T2>T1, the correct statement(s) is/are:
(Assume △Hθ and △Sθ are independent of temperature and ratio of lnK at T1 to lnK at T2 is greater than T2T1. Here H, S, G and K are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)

- △Hθ<0, △Sθ<0
- △Gθ<0, △Hθ>0
- △Gθ<0, △Sθ<0
- △Gθ<0, △Sθ>0
View Solution
Q. To measure the quantity of MnCl2 dissolved in an aqueous solution, it was completely converted to KMnO4 using the reaction, MnCl2+K2S2O8+H2O→KMnO4+H2SO4+HCl (equation not balanced).
Few drops of concentrated HCl were added to this solution and gently warmed. Further, oxalic acid (225 g) was added in portions till the colour of the permanganate ion disappeared. The quantity of MnCl2 (in mg) present in the initial solution is:
(Atomic weights in g mol−1:Mn=55, Cl=35.5).
Few drops of concentrated HCl were added to this solution and gently warmed. Further, oxalic acid (225 g) was added in portions till the colour of the permanganate ion disappeared. The quantity of MnCl2 (in mg) present in the initial solution is:
(Atomic weights in g mol−1:Mn=55, Cl=35.5).
View Solution
Q. The total number of compounds having at least one bridging oxo group among the molecules given below is:
N2O3, N2O5, P4O6, P4O7, H4P2O5, H5P3O10, H2S2O3, H2S2O5.
N2O3, N2O5, P4O6, P4O7, H4P2O5, H5P3O10, H2S2O3, H2S2O5.
View Solution