1
Question
What is the metallic radius?
What is the metallic radius?
Open in App
Solution
Part 1: Definition of metallic radius:
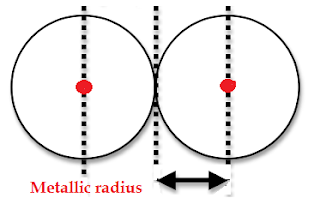
- It is half the distance between the nuclei of two metal atoms in a metallic structure.
- The atoms are joined by metallic bonds in this kind of metallic structure.
Part 2: Trend of metallic radius in the periodic table:
- On moving across a period from left to right, the metallic radius generally decreases due to an increase in the effective nuclear charge.
- On moving from top to bottom in a group, the metallic radius generally increases due to an increase in principal quantum number.
Part 1: Definition of metallic radius:
- It is half the distance between the nuclei of two metal atoms in a metallic structure.
- The atoms are joined by metallic bonds in this kind of metallic structure.
Part 2: Trend of metallic radius in the periodic table:
- On moving across a period from left to right, the metallic radius generally decreases due to an increase in the effective nuclear charge.
- On moving from top to bottom in a group, the metallic radius generally increases due to an increase in principal quantum number.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
