1
Question
What type of bonding would you expect between the following?
(i) Magnesium and chlorine in magnesium chloride.
What type of bonding would you expect between the following?
(i) Magnesium and chlorine in magnesium chloride.
Open in App
Solution
Type of Bonding = Ionic bonding
1. Ionic compounds are formed by the transfer of electrons.
2. They have strong intermolecular forces of attraction.
3. They have high melting and boiling point.
4. Bonding involves metal and non-metal.
structure of Magnesium chloride-
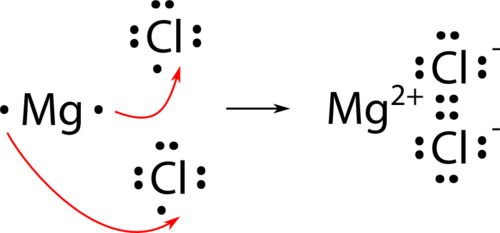
Type of Bonding = Ionic bonding
1. Ionic compounds are formed by the transfer of electrons.
2. They have strong intermolecular forces of attraction.
3. They have high melting and boiling point.
4. Bonding involves metal and non-metal.
structure of Magnesium chloride-

Suggest Corrections
0
