77
Question
Which of the following does not obey the octet or duplet rule?
(i) H2O (ii) BF3 (iii) PCl5(iv) CO2 (v) NH3
Which of the following does not obey the octet or duplet rule?
(i) H2O (ii) BF3 (iii) PCl5(iv) CO2 (v) NH3
Open in App
Solution
The correct option is B (ii), (iii)
(i) In H2O, O atom has 8 electrons in its valence shell and H atoms have two electrons.

(ii) The electronic configuration of B is:
1s22s22p1. In BF3, each F atom shares one electron with boron atom. Thus, boron atom in BF3 has 6 valence electrons. So, it does not satisfy the octet rule.
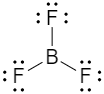
(iii) The electronic configuration of P is: 1s22s22p63s23p3. In PCl5, each Cl atom shares one electron with P - atom. Thus, phosphorous atom in PCl5 has 10 valence electrons, so it does not satisfy the octet rule.

(iv) In CO2, both C and O have 8 valence electrons. Thus, it satisfies octet rule.

(v) In NH3, N -atom has 8 valence electrons and H has two valence electrons.

Thus, BF3 and PCl5 do not follow octet rule.
(i) In H2O, O atom has 8 electrons in its valence shell and H atoms have two electrons.

(ii) The electronic configuration of B is:
1s22s22p1. In BF3, each F atom shares one electron with boron atom. Thus, boron atom in BF3 has 6 valence electrons. So, it does not satisfy the octet rule.

(iii) The electronic configuration of P is: 1s22s22p63s23p3. In PCl5, each Cl atom shares one electron with P - atom. Thus, phosphorous atom in PCl5 has 10 valence electrons, so it does not satisfy the octet rule.

(iv) In CO2, both C and O have 8 valence electrons. Thus, it satisfies octet rule.

(v) In NH3, N -atom has 8 valence electrons and H has two valence electrons.

Thus, BF3 and PCl5 do not follow octet rule.
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
