175
Question
which substance is oxidized, reduced, and oxidizing agent?
which substance is oxidized, reduced, and oxidizing agent?
Open in App
Solution
- The given reaction .
- The oxidation state of elements in reaction:
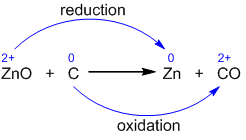
- So, carbon is oxidized because there is an increase in its oxidation state, while zinc is reduced because there is a decrease in its oxidation state.
- The above reaction is an example of a redox reaction because carbon is oxidized and zinc is getting reduced simultaneously.
Changes in oxidation state
- gets oxidized from the oxidation state .
- gets reduced from the oxidation state .
- An oxidizing agent is .
- The given reaction .
- The oxidation state of elements in reaction:
- So, carbon is oxidized because there is an increase in its oxidation state, while zinc is reduced because there is a decrease in its oxidation state.
- The above reaction is an example of a redox reaction because carbon is oxidized and zinc is getting reduced simultaneously.
Changes in oxidation state
- gets oxidized from the oxidation state .
- gets reduced from the oxidation state .
- An oxidizing agent is .
Suggest Corrections
0
Join BYJU'S Learning Program
Join BYJU'S Learning Program
