The human body is composed of different types of cells, tissues and other complex organs. For efficient functioning, our body releases some chemicals to accelerate biological processes such as respiration, digestion, excretion and few other metabolic activities to sustain a healthy life. Hence, enzymes are pivotal in all living entities which govern all the biological processes.
Table of Contents
Let us understand what are enzymes, types, their structure, mechanism and various factors that affect its activity.
What Are Enzymes?
“Enzymes can be defined as biological polymers that catalyze biochemical reactions.”
Majority of enzymes are proteins with catalytic capabilities crucial to perform different processes. Metabolic processes and other chemical reactions in the cell are carried out by a set of enzymes that are necessary to sustain life.
The initial stage of metabolic process depends upon the enzymes, which react with a molecule and is called the substrate. Enzymes convert the substrates into other distinct molecules and are called the products.
The regulation of enzymes has been a key element in clinical diagnosis because of their role in maintaining life processes. The macromolecular components of all enzymes consist of protein, except in the class of RNA catalysts called ribozymes. The word ribozyme is derived from the ribonucleic acid enzyme. Many ribozymes are molecules of ribonucleic acid, which catalyze reactions in one of their own bonds or among other RNAs.
Enzymes are found in all tissues and fluids of the body. Catalysis of all reactions taking place in metabolic pathways are carried out by intracellular enzymes. The enzymes in plasma membrane govern the catalysis in the cells as a response to cellular signals and enzymes in the circulatory system regulate clotting of blood. Most of the critical life processes are established on the functions of enzymes.
Enzyme Structure
Enzymes are a linear chain of amino acids, which give rise to a three-dimensional structure. The sequence of amino acids specifies the structure, which in turn identifies the catalytic activity of the enzyme. Upon heating, enzyme’s structure denatures, resulting in a loss of enzyme activity, that typically is associated with temperature.
Compared to its substrates, enzymes are typically large with varying sizes, ranging from 62 amino acid residues to an average of 2500 residues found in fatty acid synthase. Only a small section of the structure is involved in catalysis and is situated next to the binding sites. The catalytic site and binding site together constitute the enzyme’s active site. A small number of ribozymes exist which serve as an RNA-based biological catalyst. It reacts in complex with proteins.
Also read: Amino acids
Enzymes Classification

Earlier, enzymes were assigned names based on the one who discovered it. With further researches, classification became more comprehensive.
According to the International Union of Biochemists (I U B), enzymes are divided into six functional classes and are classified based on the type of reaction in which they are used to catalyze. The six kinds of enzymes are hydrolases, oxidoreductases, lyases, transferases, ligases and isomerases.
Listed below is the classification of enzymes discussed in detail:
| Types | Biochemical Property |
| Oxidoreductases | The enzyme Oxidoreductase catalyzes the oxidation reaction where the electrons tend to travel from one form of a molecule to the other. |
| Transferases | The Transferases enzymes help in the transportation of the functional group among acceptors and donor molecules. |
| Hydrolases | Hydrolases are hydrolytic enzymes, which catalyze the hydrolysis reaction by adding water to cleave the bond and hydrolyze it. |
| Lyases | Adds water, carbon dioxide or ammonia across double bonds or eliminate these to create double bonds. |
| Isomerases | The Isomerases enzymes catalyze the structural shifts present in a molecule, thus causing the change in the shape of the molecule. |
| Ligases | The Ligases enzymes are known to charge the catalysis of a ligation process. |
Oxidoreductases
These catalyze oxidation and reduction reactions, e.g. pyruvate dehydrogenase, catalysing the oxidation of pyruvate to acetyl coenzyme A.
Transferases
These catalyze transferring of the chemical group from one to another compound. An example is a transaminase, which transfers an amino group from one molecule to another.
Hydrolases
They catalyze the hydrolysis of a bond. For example, the enzyme pepsin hydrolyzes peptide bonds in proteins.
Lyases
These catalyze the breakage of bonds without catalysis, e.g. aldolase (an enzyme in glycolysis) catalyzes the splitting of fructose-1, 6-bisphosphate to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.
Isomerases
They catalyze the formation of an isomer of a compound. Example: phosphoglucomutase catalyzes the conversion of glucose-1-phosphate to glucose-6-phosphate (phosphate group is transferred from one to another position in the same compound) in glycogenolysis (glycogen is converted to glucose for energy to be released quickly).
Ligases
Ligases catalyze the association of two molecules. For example, DNA ligase catalyzes the joining of two fragments of DNA by forming a phosphodiester bond.
Cofactors
Cofactors are non-proteinous substances that associate with enzymes. A cofactor is essential for the functioning of an enzyme. An enzyme without a cofactor is called an apoenzyme. An enzyme and its cofactor together constitute the holoenzyme.
There are three kinds of cofactors present in enzymes:
- Prosthetic groups: These are cofactors tightly bound to an enzyme at all times. A fad is a prosthetic group present in many enzymes.
- Coenzyme: A coenzyme binds to an enzyme only during catalysis. At all other times, it is detached from the enzyme. NAD+ is a common coenzyme.
- Metal ions: For the catalysis of certain enzymes, a metal ion is required at the active site to form coordinate bonds. Zn2+ is a metal ion cofactor used by a number of enzymes.
Examples of Enzymes
Following are some of the examples of enzymes:
Beverages
Alcoholic beverages generated by fermentation vary a lot based on many factors. Based on the type of the plant’s product, which is to be used and the type of the enzyme applied, the fermented product varies.
For example, grapes, honey, hops, wheat, cassava roots, and potatoes depending upon the materials available. Beer, wines and other drinks are produced from plant fermentation.
Food Products
Bread can be considered as the finest example of fermentation in our everyday life.
A small proportion of yeast and sugar is mixed with the batter for making bread. Then one can observe that the bread gets puffed up as a result of fermentation of the sugar by the enzyme action in yeast, which leads to the formation of carbon dioxide gas. This process gives the texture to the bread, which would be missing in the absence of the fermentation process.
Drug Action
Enzyme action can be inhibited or promoted by the use of drugs which tend to work around the active sites of enzymes.
Also Read: Digestive Enzymes
Mechanism of Enzyme Reaction
Any two molecules have to collide for the reaction to occur along with the right orientation and a sufficient amount of energy. The energy between these molecules needs to overcome the barrier in the reaction. This energy is called activation energy.
Enzymes are said to possess an active site. The active site is a part of the molecule that has a definite shape and the functional group for the binding of reactant molecules. The molecule that binds to the enzyme is referred to as the substrate group. The substrate and the enzyme form an intermediate reaction with low activation energy without any catalysts.

The basic mechanism of enzyme action is to catalyze the chemical reactions, which begins with the binding of the substrate with the active site of the enzyme. This active site is a specific area that combines with the substrate.
Enzyme-Substrate Interactions
Enzymes are the biocatalysts with high molecular weight proteinous compound. It enhances the reactions which occur in the body during various life processes. It helps the substrate by providing the surface for the reaction to occur. The enzyme comprises hollow spaces occupying groups such as -SH, -COOH, and others on the outer surface. The substrate which has an opposite charge of the enzyme fits into these spaces, just like a key fits into a lock. This substrate binding site is called the active site of an enzyme (E).
The favourable model of enzyme-substrate interaction is called the induced-fit model. This model states that the interaction between substrate and enzyme is weak, and these weak interactions induce conformational changes rapidly and strengthen binding and bring catalytic sites close enough to substrate bonds.
There are four possible major mechanisms of catalysis:
Catalysis by Bond Strain
The induced structural rearrangements in this type of catalysis produce strained substrate bonds that attain transition state more easily. The new conformation forces substrate atoms and catalytic groups like aspartate into conformations that strain substrate bonds.
Covalent Catalysis
The substrate is oriented to active place on the enzymes in such a manner that a covalent intermediate develops between the enzyme and the substrate, in catalysis that occurs by covalent mechanisms. The best example of this involves proteolysis by serine proteases that have both digestive enzymes and various enzymes of the blood clotting cascade. These proteases possess an active site serine whose R group hydroxyl generates a covalent bond with a carbonyl carbon of a peptide bond and results in the hydrolysis of the peptide bond.
Catalysis Involving Acids and Bases
Other mechanisms add to the completion of catalytic events which are launched by strain mechanisms such as the usage of glutamate as a general acid catalyst.
Catalysis by Orientation and Proximity
Enzyme-substrate interactions induce reactive groups into proximity with one another. Also, groups like aspartate are chemically reactive, and their proximity towards the substrate favours their involvement in catalysis.
Action and Nature of Enzymes
Once substrate (S) binds to this active site, they form a complex (intermediate-ES) which then produces the product (P) and the enzyme (E). The substrate which gets attached to the enzyme has a specific structure and that can only fit in a particular enzyme. Hence, by providing a surface for the substrate, an enzyme slows down the activation energy of the reaction. The intermediate state where the substrate binds to the enzyme is called the transition state. By breaking and making the bonds, the substrate binds to the enzyme (remains unchanged), which converts into the product and later splits into product and enzyme. The free enzymes then bind to other substrates and the catalytic cycle continues until the reaction completes.
The enzyme action basically happens in two steps:
Step1: Combining of enzyme and the reactant/substrate.
E+S → [ES]
Step 2: Disintegration of the complex molecule to give the product.
[ES]→E+P
Thus, the whole catalyst action of enzymes is summarized as:
E + S → [ES] → [EP] → E + P
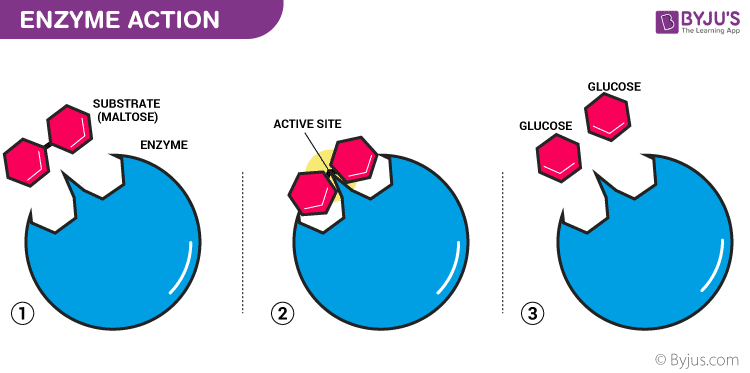
Biological Catalysts
Catalysts are the substances which play a significant role in the chemical reaction. Catalysis is the phenomenon by which the rate of a chemical reaction is altered/ enhanced without changing themselves. During a chemical reaction, a catalyst remains unchanged, both in terms of quantity and chemical properties. An enzyme is one such catalyst which is commonly known as the biological catalyst. Enzymes present in the living organisms enhance the rate of reactions which take place within the body.
Biological catalysts, enzymes, are extremely specific that catalyze a single chemical reaction or some closely associated reactions. An enzyme’s exact structure and its active site decide an enzyme’s specificity. Substrate molecules attach themselves at the active site of an enzyme. Initially, substrates associate themselves by noncovalent interactions to the enzymes which include ionic, hydrogen bonds and hydrophobic interactions. Enzymes reduce the reactions and activation energy to progress towards equilibrium quicker than the reactions that are not catalyzed. Both eukaryotic and prokaryotic cells usually make use of allosteric regulation to respond to fluctuations in the state inside the cells.
The nature of enzyme action and factors affecting the enzyme activity are discussed below.
Factors Affecting Enzyme Activity
The conditions of the reaction have a great impact on the activity of the enzymes. Enzymes are particular about the optimum conditions provided for the reactions such as temperature, pH, alteration in substrate concentration, etc.

Typically, enzyme activities are accelerated with increasing temperatures. As enzymes are functional in cells, the feasible conditions for nearly all enzymes are temperatures that are moderate. At higher temperatures, given a specific point, there is a drastic decrease in the activity with the denaturation of enzymes. In diluted solutions, purified enzymes denature quickly compared to enzymes in crude extracts. Denaturation of enzymes can also take place when enzymes are incubated for long durations. More appropriate is to utilize a shorter time duration when it comes to incubation time to gauge the starting velocities of such enzyme reactions.
The International Union of Biochemistry suggests the standard assay temperature to be 30 °C. Almost all enzymes are extremely sensitive to pH change. Just some enzymes feasibly operate with pH above 9 and below 5. Most enzymes have their pH – optimum near to neutrality. Any alteration of pH causes the ionic state of amino acid residues to change in the whole protein and in the active site. The modifications in the ionic state can modify catalysis and substrate binding. The preference of substrate concentration is critical as at lower concentrations, the rate is driven by concentration, however, at high concentrations, the rate does not depend on any increase in the concentration of the substrate.
Active site
Enzymatic catalysis depends upon the activity of amino acid side chains assembled in the active centre. Enzymes bind the substrate into a region of the active site in an intermediate conformation.
Often, the active site is a cleft or a pocket produced by the amino acids which take part in catalysis and substrate binding. Amino acids forming an enzyme’s active site is not contiguous to the other along the sequence of primary amino acid. The active site amino acids are assembled to the cluster in the right conformation by the 3-dimensional folding of the primary amino acid sequence. The most frequent active site amino acid residues out of the 20 amino acids forming the protein are polar amino acids, aspartate, cysteine, glutamate, histidine, Serine, and lysine. Typically, only 2-3 essential amino acid residues are involved directly in the bond causing the formation of the product. Glutamate, Aspartate, and histidine are the amino acid residues which also serve as a proton acceptor or donor.
Temperature and pH
Enzymes require an optimum temperature and pH for their action. The temperature or pH at which a compound shows its maximum activity is called optimum temperature or optimum pH, respectively. As mentioned earlier, enzymes are protein compounds. A temperature or pH more than optimum may alter the molecular structure of the enzymes. Generally, an optimum pH for enzymes is considered to be ranging between 5 and 7.
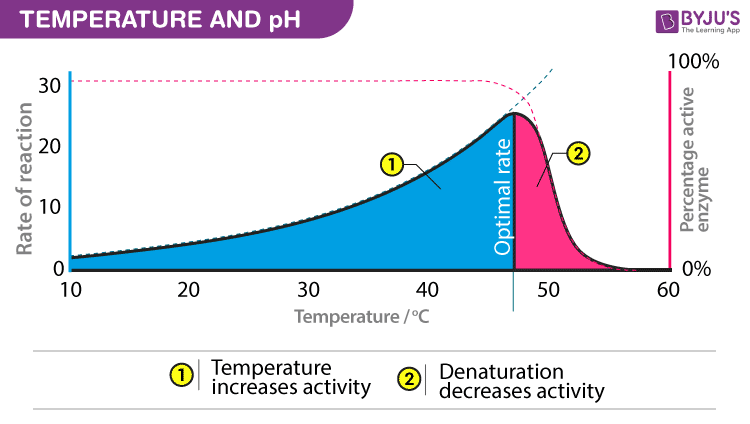
- Optimum T°
- The greatest number of molecular collisions
- human enzymes = 35°- 40°C
- body temp = 37°C
- Heat: increase beyond optimum T°
- The increased energy level of molecule disrupts bonds in enzyme & between enzyme & substrate H, ionic = weak bonds
- Denaturation = lose 3D shape (3° structure)
- Cold: decrease T°
- Molecules move slower decrease collisions between enzyme & substrate
Concentration and Type of Substrate
Enzymes have a saturation point, i.e., once all the enzymes added are occupied by the substrate molecules, its activity will be ceased. When the reaction begins, the velocity of enzyme action keeps on increasing on further addition of substrate. However, at a saturation point where substrate molecules are more in number than the free enzyme, the velocity remains the same.
The type of substrate is another factor that affects the enzyme action. The chemicals that bind to the active site of the enzyme can inhibit the activity of the enzyme and such substrate is called an inhibitor. Competitive inhibitors are chemicals that compete with the specific substrate of the enzyme for the active site. They structurally resemble the specific substrate of the enzyme and bind to the enzyme and inhibit the enzymatic activity. This concept is used for treating bacterial infectious diseases.
Salt concentration
Changes in salinity: Adds or removes cations (+) & anions (–)
- Disrupts bonds, disrupts the 3D shape
- Disrupts attractions between charged amino acids
- Affect 2° & 3° structure
- Denatures protein
- Enzymes intolerant of extreme salinity
- The Dead Sea is called dead for a reason
Functions of Enzymes
The enzymes perform a number of functions in our bodies. These include:
- Enzymes help in signal transduction. The most common enzyme used in the process includes protein kinase that catalyzes the phosphorylation of proteins.
- They break down large molecules into smaller substances that can be easily absorbed by the body.
- They help in generating energy in the body. ATP synthase is the enzymes involved in the synthesis of energy.
- Enzymes are responsible for the movement of ions across the plasma membrane.
- Enzymes perform a number of biochemical reactions, including oxidation, reduction, hydrolysis, etc. to eliminate the non-nutritive substances from the body.
- They function to reorganize the internal structure of the cell to regulate cellular activities.
Learn more about Enzymes, its structure, classification, functions and other related topics at BYJU’S Biology



Very helpful. Great work.
Bundle of Thanks
Useful
Very comprehensive and very informative thank you for this, very useful
Great explanation !!!!!!!!
great explaanation!
It is so helpful
useful information
Very good work
It is very Nice
This is very helpful and cleared all of my concepts about enzymes.
Nyc work I understand ☺️
good explanation
I like to take the notes
This is very helpful and cleared all of my concepts. ?