Calcium is an essential metal for living organisms. Due to its chemical properties, it finds usage in the preparation of alloys as a deoxidizer, desulfurizer and decarbonizer. However, it is not just the metal itself, a number of calcium compounds are also crucial to several industries and are prepared on a large scale.
Some of them are calcium carbonate, calcium oxide (quick lime), calcium hydroxide, calcium sulphate, calcium chloride, etc.

What is Calcium Sulphate?
- It is a calcium compound with formula CaSO4 and its related hydrates.
- Its forms are white solids, barely soluble in water.
- It’s two common hydrates are Plaster of Paris and Gypsum.
- Plaster of Paris is a Hemi-hydrate of calcium sulphate which is obtained by heating gypsum to 393 K.
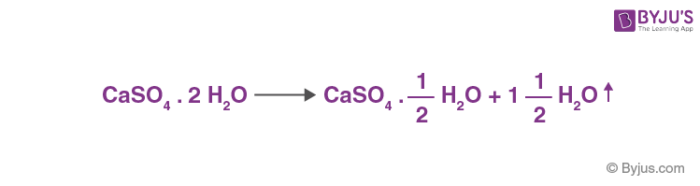
- If it is heated above 393 K, it forms anhydrite, also known as ‘dead burnt plaster’, which will again form gypsum on mixing with water.
- It is used in building industries, for the construction of false ceilings, like plasters, etc.
- In art, it is used for making statues and burial sites.
- In medicine, it is used indentures.
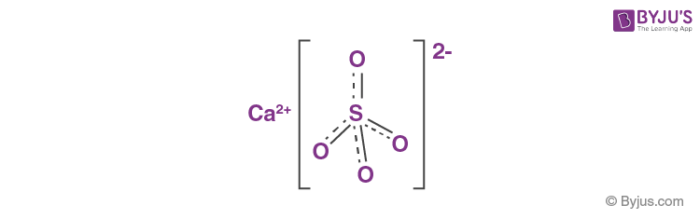
Hydration States
- CaSO4 (anhydrite): the anhydrous state.
- CaSO4 · 2 H2O (gypsum): dihydrate.
- CaSO4 · 0.5 H2O (bassanite): hemihydrate, also known as plaster of Paris. Specific hemihydrates are sometimes distinguished: alpha-hemihydrate and beta-hemihydrate.
Uses of Calcium Sulphate
- Its major use is in the manufacture of Plaster of Paris. Due to its ability to be moulded in the paste form on applying water to the powdered form of Calcium Sulphate.
- Calcium Sulphate is used in tofu as a coagulant.
- The compound was used to make sulphuric acid before the 1970s.
- Used as a moisture indicator.
To learn more about calcium carbonate, calcium oxide and other calcium compounds, download BYJU’S The Learning App.




Comments