What is a Carboxyl Group?
A Carboxyl Group is a functional organic compound that comprises a double-bonded carbon atom linked to an oxygen group and a hydroxyl group through a single bond. An organic compound consisting of a carboxyl group is termed as a carboxylic acid. They include acetic acid and amino acid.
Formula of Carboxyl group
The formula for carboxyl group is R-COOH wherein R is the organic compound chain.
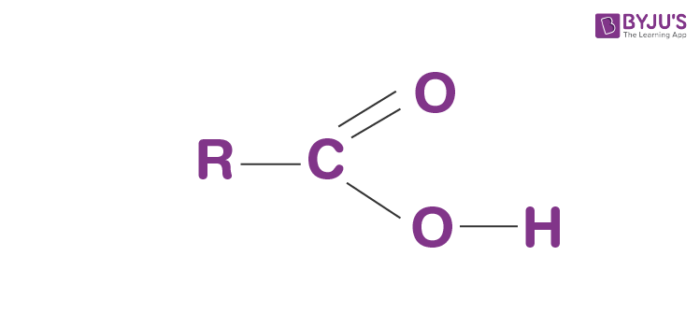
Structure of Carboxyl group
Carboxyl groups are present on the side of a molecule. It ionizes, discharging the H from the hydroxyl aggregate as a free proton (H+), with the rest of the O conveying a -ve charge. This charge turns about forward and backwards between the 2 oxygen molecules, which makes this ionized state moderately steady.
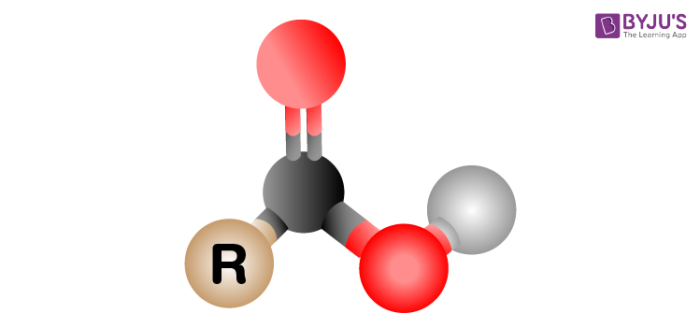
Properties of Carboxyl Group
- Carboxyl group comprises electronegative oxygen double bond to a carbon atom. As a result, there will be an increase in the polarity of a bond.
- A compound comprising a carboxyl group will possess a high melting point, hydrophilic centres, and boiling point.
- The reason behind high melting and boiling point can be dissipated because of the formation of a hydrogen bond in the solid and liquid state. One of the typical examples is fatty acids.
Carboxyl Group From Nature
Acetic acid can be naturally blended either by anaerobic fermentation, the procedure used to make vinegar. The rigorous method requires warm ethanol and oxygen with Acetobacter. The anaerobic process requires just sugar as an input chemical, and acetogen can then give carboxylic acids as yield. It ought to be noticed that the high-impact process is still predominantly connected due to acetogenins utilized for anaerobic procedures indicating less resistance to acidic situations. At the end of the day, acetogenesis will be destroyed if a lot of acids are produced to a great volume.
Benzoic acid is a fundamental part of benzoin resin. It is genuinely expensive to remove benzoic acid specifically from benzoin resins. Along these lines, most of the benzoic acid in the market is produced mechanically.
Register with BYJU’S for more learning content.



Comments