What is Eugenol?
Eugenol is a weakly acidic phenolic compounds with a chemical formula C10H12O2.
Eugenol is an allylic substituted guaiacol which is pale yellow oily liquid. It is one of the most important compounds present in clove has a pleasant, spicy, clove like odour. Eugenol (4-allyl-2-methoxyphenol) exhibits antioxidant, antiseptic, and antimicrobial activity properties. Eugenol is one of the reduced phenylpropanoids, a major component of clove oil.
Other name – 2-methoxy-4-prop-2-enylphenol, 4-Allyl-2-methoxyphenol
| C10H12O2 | Eugenol |
| Density | 1.06 g/cm³ |
| Molecular Weight/ Molar Mass | 164.2 g/mol |
| Boiling Point | 254 °C |
| Melting Point | -7.5 °C |
| Chemical Formula | C10H12O2 |
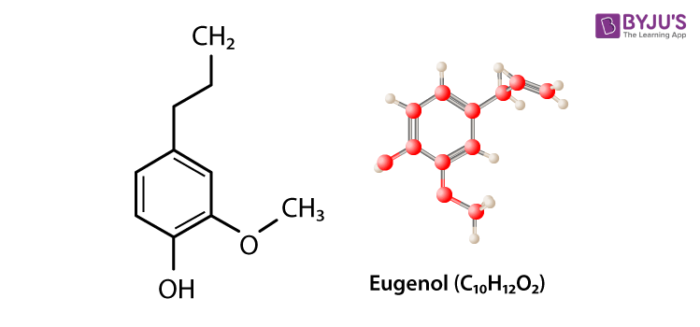
Eugenol Structure – C10H12O2
Physical Properties of Eugenol – C10H12O2
| Odour | Odor of cloves |
| Appearance | Pale yellow aromatic oily liquid |
| Complexity | 145 |
| Vapour Pressure | Approximately 4 torr |
| Hydrogen Bond Donor | 1 |
| Solubility | Miscible with alcohol, ether, chloroform and oils |
Chemical Properties of Eugenol – C10H12O2
-
- Eugenol readily undergo double displacement reaction with Iron(III) chloride. The reaction is as below.
C10H12O2 + FeCl3 → C10H9O2Fe + 3HCl
-
- Eugenol reacts with bromine forms 1,3 – Dibromobutyl benzene and oxygen. The chemical reaction is as follows.
C10H12O2 + Br2 → C10H12Br2 + O2
Uses of Eugenol – C10H12O2
- Used as Food Flavouring Clove is very aromatic, has a fine flavour and imparts warming qualities.
- Used as an inhibitor for additional polymerizing resins and can interfere with subsequent use of resin cements.
- Used as a dental obtundent by dentist and as a topical anesthetic used extensively to replace clove oil.
- Zinc oxide eugenol cement is one of the oldest used cements. It is only a mild irritant to the pulp, less soluble in oral fluids and produce better marginal seal.
Frequently Asked Questions
What is the use of eugenol?
Dentists usually use clove oil or eugenol, as it is antiseptic and anti-inflammatory. You also apply it to the gums to remove germs and alleviate dental treatment discomfort such as extractions of the dentures, fillings, and root canals.
Does eugenol kill bacteria?
Eugenol is one of the planet’s most potent antioxidants. It is also a natural antibacterial and antimicrobial which kills bacteria, viruses and other pathogenic substances. One research reveals that Eugenol prevents the development of more than 25 different harmful bacteria including candida and salmonella.
Is eugenol toxic?
Unlike most essential oils and their main constituents, clove oil and eugenol can be toxic when ingested internally or when absorbed through the skin at relatively low concentrations (never apply essential oils directly to the skin). Eugenol makes internal use of the professional supervision.
Why eugenol is not soluble in water?
Eugenol dissolves with methylene chloride but not in water due to non-polar eugenol and polar water and polar methyl chloride. Through distillation, the eugenol oil was isolated from the clove, and then removed.
Which essential oils contain eugenol?
Particularly from clove oil, nutmeg, cinnamon, basil and bay leaf, it is a colorless to pale yellow, aromatic oily liquid extracted from some essential oils. This is present in 80–90% concentrations in clove bud oil, and 82–88% in clove leaf oil. Eugenol has a sweet, aromatic, clove-like scent.




Comments