Grignard reagents are extremely useful organometallic compounds in the field of organic chemistry. They exhibit strong nucleophilic qualities and also have the ability to form new carbon-carbon bonds. Therefore, they display qualities that are also exhibited by organolithium reagents and the two reagents are considered similar.
When the alkyl group attached to a Grignard reagent is replaced by an amido group, the resulting compound is called a Hauser base. These compounds are even more nucleophilic that their Grignard counterparts.
Table of Contents
- What are Grignard Reagents
- Grignard Reagents
- Preparation of Grignard Reagents
- Recommended Videos
- Reactions of Grignard Reagents
- Frequently Asked Questions – FAQs
Grignard reagents are known for their ability to quickly target carbonyls at their carbon level. Grignard reagents do not, however, function in the presence of protic solvents. Instead of reacting with the desired molecule, the Grignard is so unstable that it can readily accept a proton from a protic solvent. The Grignard then is inert and there is no reaction to the desired molecule.
What are Grignard Reagents?
A Grignard reagent is an organomagnesium compound which can be described by the chemical formula ‘R-Mg-X’ where R refers to an alkyl or aryl group and X refers to a halogen.
They are generally produced by reacting an aryl halide or an alkyl halide with magnesium.
These reagents were discovered by the French chemist Victor Grignard, who won the Nobel Prize in Chemistry in the year 1912 for his work on these compounds.
Grignard Reagents
Grignard reagents (RMgX) are commonly used for organic synthesis. However, these highly reactive compounds are supplied in flammable solvents, which cause extra complexity in their transport. Herein we note that Grignard reagents with linear alkyl chains can be trapped and stabilized by the macrocyclic host pillar arene while retaining their reactivity.
Reactions that form carbon-carbon bonds are among the most beneficial to the synthetic organic chemist. In 1912, Victor Grignard was awarded the Nobel Prize in Chemistry for his discovery of a new sequence of reactions resulting in the creation of a carbon-carbon bond. Grignard synthesis involves the preparation of an organomagnesium reagent through the reaction of an alkyl bromide with magnesium metal.
The Grignard reaction is an organic reaction used to produce a variety of products through the reaction of an organomagnesium compound, also known as an electrophilic “Grignard reagent,” followed by an acidic reaction. The Grignard reagent is formed by the reaction of an alkyl or aryl halide with magnesium metal via a radical mechanism.
Preparation of Grignard Reagents
The process of preparing Grignard reagents is described in the points provided below. It can be noted that many of these reagents can also be purchased commercially.
- These reagents are prepared via the treatment of magnesium with organic halides such as alkyl or aryl halides.
- This is done with the help of solvents comprising ethers (which are described by the formula R-O-R’) because the ligands provided by these solvents help in the stabilization of the organomagnesium compound.
- Water and air are very harmful to this synthesis and can quickly destroy the Grignard reagent which is being formed via protonolysis or via oxidation of the reagent. Therefore, the process must be carried out in air-free conditions.
- Alternatively, the magnesium can be activated to make it consume water when wet solvents are used with the help of ultrasound.
- After the slow induction period of the reaction, the process can be quite exothermic. This is a very important factor to consider while industrially producing the Grignard reagent.
- The organic halides used in these reactions include aryl or alkyl chlorides, bromides, and iodides. Aryl fluorides and alkyl fluorides are not very reactive and are hence not commonly used. However, with the help of Rieke metals, the magnesium can be activated to make the fluoride more reactive.
An illustration detailing the preparation of these reagents is provided below.
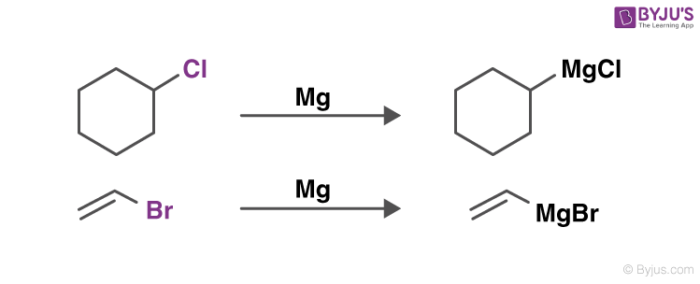
The quality testing of the synthesized Grignard reagents is done via titrations involving protic reagents that do not contain water (since these reagents are highly sensitive to oxygen and water) and a colour indicator. One suitable compound for these titrations is methanol.
Recommended Videos

Reactions of Grignard Reagents
During a reaction involving Grignard reagents, it is necessary to ensure that no water is present which would otherwise cause the reagent to decompose rapidly. Therefore, the majority of Grignard reactions occur in solvents such as anhydrous diethyl ether or tetrahydrofuran because the oxygen in these solvents stabilizes the magnesium reagent.
Grignard reagents are very important reagents in organic chemistry since they can be reacted with a wide range of compounds to form different products. Some of these reactions of these reagents are listed below.
1. Reactions with Carbonyl Group
These reagents form various products when reacted with different carbonyl compounds. The most common reaction of Grignard reagents is the alkylation of ketones and aldehydes with the help of R-Mg-X.
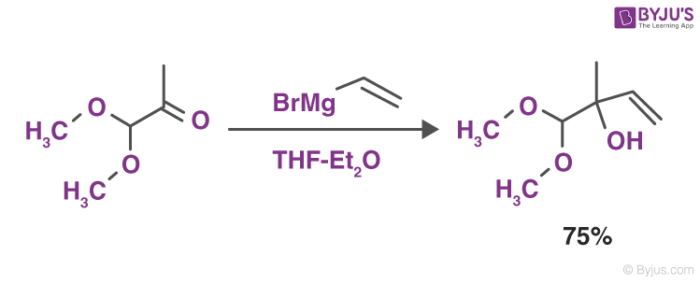
This reaction depicted above is also referred to as the Grignard reaction. The solvents that are used in this reaction include tetrahydrofuran and diethyl ether.
2. Reactions with Non-Carbon Electrophiles
For the formation of new carbon-heteroatom bonds, Grignard reagents and some organolithium compounds are very useful. These reagents can also undergo a transmetallation reaction with cadmium chloride, yielding dialkyl cadmium. This reaction can be written as follows.
2R-Mg-X + CdCl2 → R2Cd + 2Mg(X)Cl
Alkyl chains can be attached to many metals and metalloids with the help of these reagents.
3. Reactions with Organic Halides
Typically, these reagents are quite unreactive towards organic halides which highly contrasts their behaviour towards other halides. However, carbon-carbon coupling reactions occur with Grignard reagents acting as a reactant when a metal catalyst is introduced.
An example of such a coupling reaction is the reaction between methyl p-chlorobenzoate and nonyl magnesium bromide which yields the compound p-nonyl benzoic acid in the presence of the catalyst – Tris(acetylaceto) iron(III).
4. Reaction between Acetone and Methyl Magnesium Chloride
The reaction of methyl magnesium bromide with acetone followed by hydrolysis gives tertiary alcohol. Acetone reacts with methyl magnesium bromide followed by hydrolysis to give secondary alcohols.
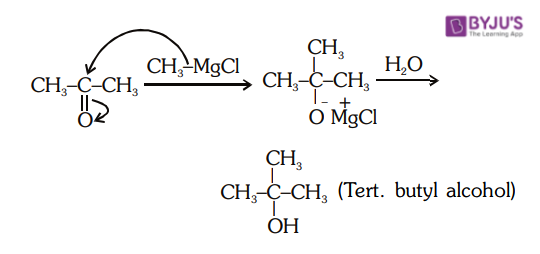
5. Industrial Reactions
For the production of Tamoxifen, a type of medication used to prevent and treat breast cancer, the Grignard reagent is a vital part of the non-stereoselective process.
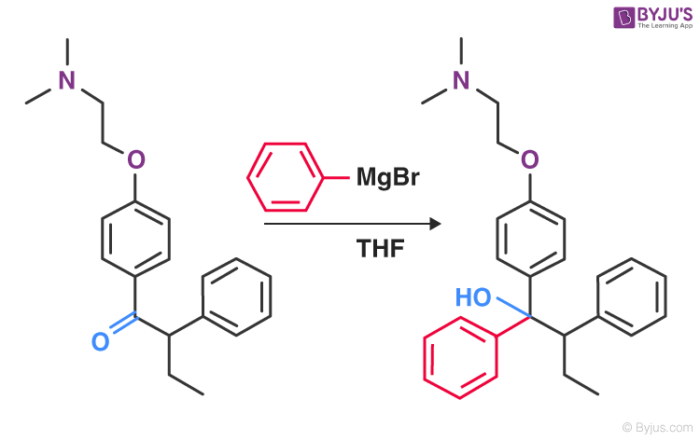
An illustration detailing the Grignard reaction that takes place in the manufacturing process of tamoxifen can be found above.
Frequently Asked Questions – FAQs
Is a Grignard reagent an organometallic compound?
Grignard reaction (pronounced / submitted / submitted/) is an organometallic chemical reaction in which alkyl, allyl, vinyl or aryl-magnesium halides (Grignard reagent) are added to the carbonyl group in aldehyde or ketone. This reaction is important for the formation of carbon-carbon bonds.
Why is mg used in Grignard reagent?
Usually, the reaction to the formation of Grignard reagents requires the use of magnesium ribbon. All magnesium is covered with a passivating film of magnesium oxide, which prevents reactions to organic halide. The application of Grignard preformed reagent is also used as an initiator.
Are Grignard reagents nucleophiles?
Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkyl halides. They are wonderful nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc.) and epoxides.
What happens when aldehyde reacts with Grignard reagent?
Organolithium or Grignard reagents react to alcohol in aldehydes or ketones with the carbonyl group C = O. Carbonyl substituents determine the essence of the alcohol component. The acidic work-up transforms the intermediate metal alkoxide salt into the desired alcohol by means of a simple acid base reaction.
Do Grignard reagents react with ethers?
The bulk of Grignard reactions are conducted in ethereal solvents, in particular diethyl ether and THF. With the chelating diether dioxane, some Grignard reagents undergo a redistribution reaction to produce organomagnesium compounds.
To learn more about the Grignard reagent and other important reagents in organic chemistry, register with BYJU’S and download the mobile application on your smartphone.




Comments