What is Osmotic Pressure?
Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). It is a colligative property and is dependent on the concentration of solute particles in the solution. Osmotic pressure can be calculated with the help of the following formula:
π = iCRT
Where,
- π is the osmotic pressure
- i is the van’t Hoff factor
- C is the molar concentration of the solute in the solution
- R is the universal gas constant
- T is the temperature
This relationship between the osmotic pressure of a solution and the molar concentration of its solute was put forward by the Dutch chemist Jacobus van’t Hoff. It is important to note that this equation only holds true for solutions that behave like ideal solutions.
Understanding Osmotic Pressure – What is Osmosis?
The term ‘osmosis’ refers to the movement of solvent molecules through a semipermeable membrane from a region where the solute concentration is low to a region where the solute concentration is high. Eventually, an equilibrium is established between the two sides of the semipermeable membrane (equal solute concentration on both sides of the semipermeable membrane).
Important note: The semipermeable membrane only allows the movement of solvent molecules through it – solute particles cannot pass through it.
If sufficient pressure is applied to the solution side of the semipermeable membrane, the process of osmosis is halted. The minimum amount of pressure required to nullify the process of osmosis is called osmotic pressure.
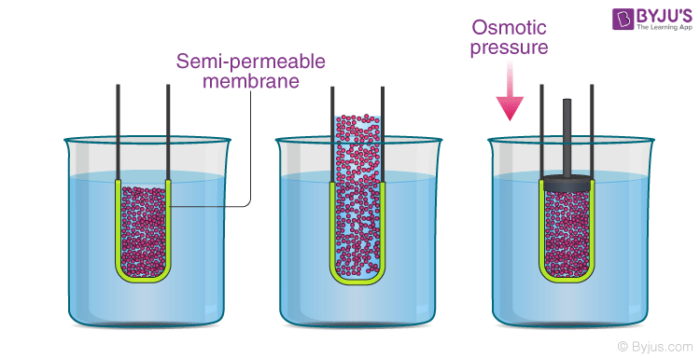
In the illustration provided above, it can be observed that the solvent molecules tend to pass through the semipermeable membrane into the solution side until the osmotic pressure (of the solution) is applied to the solution side.
What happens when Pressure of Higher Magnitude than the Osmotic Pressure is Applied to the Solution Side?
In such a scenario, the solvent molecules would start moving through the semipermeable membrane from the solution side (where the solute concentration is high) to the solvent side (where the solute concentration is low). This process is called reverse osmosis (click the hyperlink to learn more about it!).
Examples and Applications
Plants maintain their upright shape with the help of osmotic pressure. When sufficient water is supplied to the plant, its cells (which contain several salts) absorb water and expand. This expansion of plant cells increases the pressure exerted on their cell walls, causing them to stand upright.
When insufficient water is supplied to the plant, its cells become hypertonic (they shrink due to loss of water). This causes them to wilt and lose their firm, upright posture. The measurement of osmotic pressure can also be used to determine molecular weights of compounds.
Another important application of osmotic pressure is in the desalination and purification of seawater, which involves the process of reverse osmosis.
Summary
| What is osmosis? | The flow of solvent molecules through a semipermeable membrane. |
| What is a semipermeable membrane? | A membrane which only allows solvent molecules to flow through it. |
| What is the direction of solvent flow? | From the solvent side to the solution side (from the region of low solute concentration to the region of high solute concentration). |
| What is osmotic pressure? | The pressure that must be applied to halt osmosis. |
| Where must the osmotic pressure be applied? | On the solution side of the semipermeable membrane (high solute concentration). |
| What is the formula for osmotic pressure? | π = iCRT |
Solved Exercises
Exercise 1
One mole of table salt is dissolved in one litre of water. At a temperature of 27oC, what would be the osmotic temperature of this solution?
The molar concentration of table salt (sodium chloride) in the solution = 1mol/1litre = 1M
Since salt (NaCl) dissociates into two ions, the value of the van’t Hoff factor here is 2. Converting 27oC to the Kelvin scale, the required temperature becomes 300K.
Therefore, the osmotic pressure of the solution is:
π = (2) * (1 mol.L-1) * (0.0821 atm.L. mol-1.K-1) (300 K)
π = 49.26 atm.
The osmotic pressure of the 1M salt solution is 49.26 atmospheres at a temperature of 27oC.
Exercise 2
The osmotic pressure of a potassium chloride solution (at 300K) is 50 atmospheres. What is the molar concentration of potassium chloride in this solution?
Rearranging the osmotic pressure equation, the following equation can be obtained:
π = iCRT ; C = π/(iRT)
Here, the value of i is 2 (since KCl dissociates into two ions). Therefore, the molarity of KCl is:
C = (50 atm)/(2)*(0.0821 atm.L.mol-1.K-1)*(300K)
C = 50/49.26 M = 1.015 M
Therefore, the molar concentration of potassium chloride in the solution is 1.015 M.
To learn more about osmotic pressure and other colligative properties (such as boiling point elevation), register with BYJU’S and download the mobile application on your smartphone.




What is osmotic pressure
Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis).