What is Sodium dihydrogen phosphate?
Sodium dihydrogen phosphate is an inorganic monobasic sodium phosphate compound with the chemical formula NaH2PO4. Sodium dihydrogen phosphate is a derivative composed of glycerol derivatives formed by reacting mono and diglycerides that are derived from edible sources with phosphorus pentoxide followed by neutralization with sodium carbonate. Sodium dihydrogen phosphate is a soluble form of phosphate that can be administered intravenously.
Other names – Monobasic sodium phosphate, Monosodium phosphate (MSP)
| NaH2PO4 | Sodium dihydrogen phosphate |
| Density | 2.36 g/cm3 (anhydrous) |
| Molecular Weight/ Molar Mass | 119.98 g/mol |
| pKa | 6.8-7.2 |
| Melting Point | 212.0oC |
| Chemical Formula | NaH2PO4 |
Sodium dihydrogen phosphate Structure – NaH2PO4
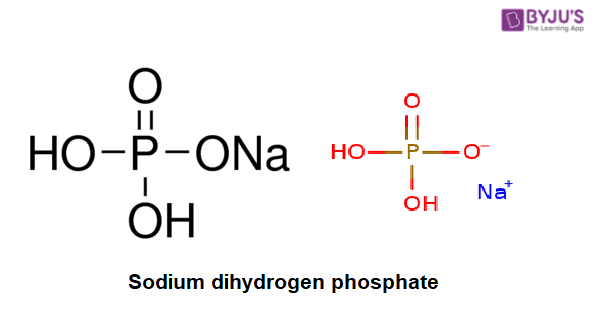
Physical Properties of Sodium dihydrogen phosphate – NaH2PO4
| Odour | Odourless |
| Appearance | White powder or crystals |
| Complexity | 61.9 |
| pH | 8.0 and 11.0 |
| Solubility in water | Soluble in water; insoluble in alcohol |
Chemical Properties of Sodium dihydrogen phosphate – NaH2PO4
-
- Sodium dihydrogen phosphate reacts with base like sodium hydroxide resulting in the formation of sodium hydrogen phosphate and water.
NaH2PO4 + NaOH → Na2HPO4 + H2O
-
- Sodium dihydrogen phosphate reacts with acid like hydrochloric acid results in the formation of phosphoric acid and sodium chloride.
NaH2PO4 + HCl → H3PO4 + NaCl
Uses of Sodium dihydrogen phosphate – NaH2PO4
- Used for water treatment were generally safe, high concentrations had the potential for interfering with human essential trace metal metabolism.
- Phosphorus in domestic sewage may be derived from human wastes, waste food (primarily from household garbage- disposal units), and synthetic detergents.
- Reducing the amount of phosphorus reaching the sewage treatment works by concerted public campaigns can reduce the costs of stripping gas or vapor from a foam.




Comments