Organic reactions are the chemical reactions that are undergone by organic compounds (the chemical compounds containing carbon). A few important types of organic reactions are illustrated below.
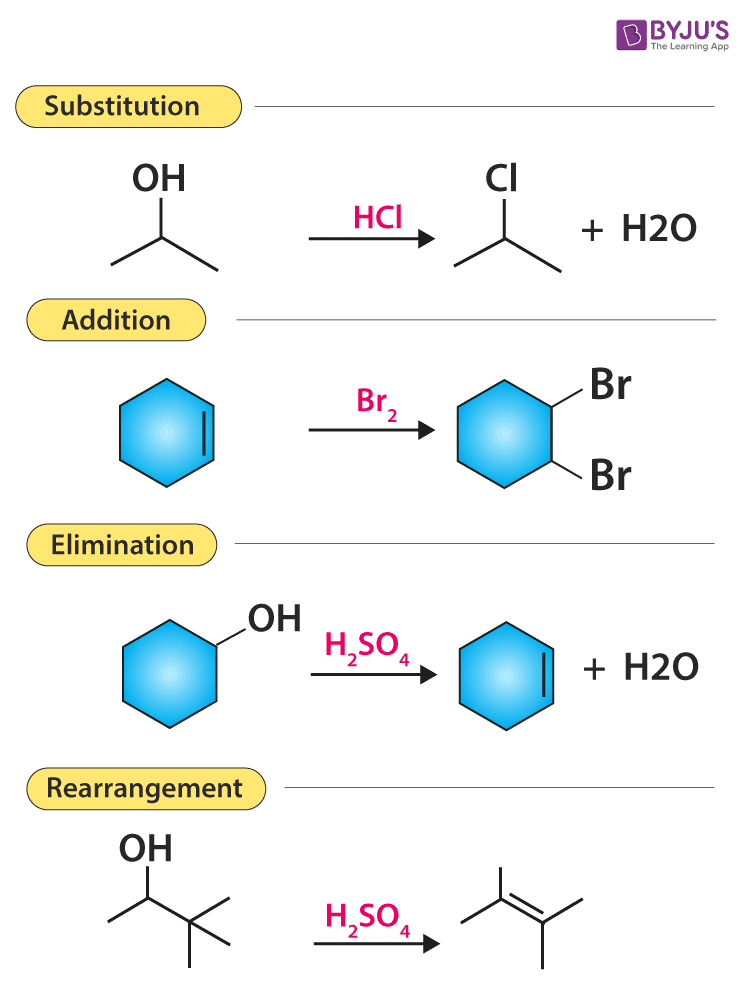
There are mainly five types of organic reactions:
- Substitution reaction
- Elimination reaction
- Addition reaction
- Radical reactions
- Oxidation-Reduction Reactions.
Substitution Reactions
In a substitution reaction, one atom or a group of atoms is substituted by another atom or a group of atoms to form a new substance.
Let us take an example of a C-Cl bond, in which the carbon atom has a partial positive charge due to the presence of highly electronegative chlorine atom. In a nucleophilic substitution reaction, the nucleophile must have a pair of electrons and also should have a high affinity for the electropositive species as compared to the substituent which was originally present.
Elimination reaction
There are some reactions which involve the elimination or removal of the adjacent atoms. After these multiple bonds are formed and there is a release of small molecules as products.
One of the examples of elimination reaction is the conversion of ethyl chloride to ethylene.
CH3CH2Cl → CH2=CH2 + HCl
In the above reaction, the eliminated molecule is HCl, which is formed by the combination of H+ from the carbon atom which is on the left side and Cl– from the carbon atom which is on the right side.
Addition reactions
Addition reaction is nothing but just the opposite of elimination reaction. In an addition reaction, the components A and B are added to the carbon-carbon multiple bonds and this is called addition reaction. In the reaction given below when HCl is added to ethylene, it will give us ethylene chloride.
HCl + CH2=CH2→ CH3CH2Cl
Also, Read: Redox Reactions
Radical reactions
Many of the organic reactions involve radicals. Addition of a halogen to a saturated hydrocarbon involves free radical mechanism. There are three stages involved in a radical reaction i.e. initiation, propagation, and termination. Initially when the weak bond is broken initiation of the reaction takes place with the formation of free radicals. After that when the halogen is added to the hydrocarbon a radical is produced and finally, it gives alkyl halide.
To learn more about organic reactions and other important concepts in organic chemistry, register with BYJU’S and download the mobile application on your smartphone.





Comments