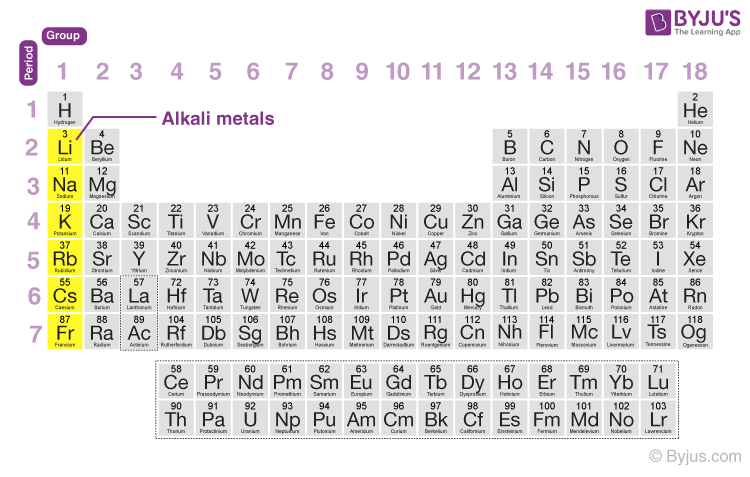
Alkali metals belong to the s-block elements occupying the leftmost side of the periodic table. Alkali metals readily lose electrons, making them count among the most reactive elements on earth. In this article, we will explain the electronic configurations, ionization enthalpy, hydration enthalpy and atomic, ionic radii and other physical and chemical properties of the group one alkali metals.
Alkali Metals Guide
- Overview
- Electronic Configuration
- Physical Properties
- Chemical Properties
- Reaction with Water
- Anomalous Behaviour
- Uses of Alkali Metals
What are Alkali Metals?
In general ‘alkali’ refers to the basic or alkaline nature of their metal hydroxides. The compounds are called alkali metals because when they react with water they usually form alkalies which are nothing but strong bases that can easily neutralize acids.
Alkali metals have a corresponding [Noble gas] ns1 electronic configuration. They occupy the first column of the periodic table. Alkali elements are Lithium(Li), Sodium(Na), Potassium (K), Rubidium (Ru), Cesium (Cs) and Francium (Fr) occupying successive periods from first to seven. Francium is a radioactive element with very low half-life.
However, the main reason why hydrogen (H) is not considered as an alkali metal is that it is mostly found as a gas when the temperature and pressure are normal. Hydrogen can show properties or transform into an alkali metal when it is exposed to extremely high pressure.
Also Read:
Alkali Metals are very reactive and are present in the form of compounds only. They are electropositive metals with unit valence.
Overview of Alkali Metals
| Metals | Lithium | Sodium | Potassium | Rubidium | Cesium |
| Atomic Number | 3 | 11 | 19 | 37 | 55 |
| Configuration | [He]2s1 | [Ne]3s1 | [Ar]4s1 | [Kr]5s1 | [Xe]6s1 |
| Abundance (ppm) | 65 | 28300 | 25900 | 310 | 7 |
| Atomic size (pm) | 152 | 186 | 227 | 248 | 265 |
| Density g/cm3 | 0.53 | 0.97 | 0.86 | 1.53 | 1.9 |
| Ionization energy kJ/mol | 520 | 496 | 419 | 403 | 376 |
| Hydration enthalpy kJ/mol | -506 | -406 | -330 | -310 | -276 |
| Reduction potential (v) | -3.04 | -2.714 | -2.925 | -2.930 | -2.927 |
| Flame colour | Crimson red | Yellow | Violet | Red Violet | Blue |
Electronic Configuration of Alkali Metals
- Alkali metals have one electron in their valence shell.
- The electronic configuration is given by ns1. For example, the electronic configuration of lithium is given by 1ns1 2ns1.
- They tend to lose the outer shell electron to form cations with charge +1 (monovalent ions).
- This makes them the most electropositive elements and due to the same reason, they are not found in the pure state.
Trends in Physical Properties of Alkali Metals
Down the column, the nuclear charge increases and a new orbital gets added to each alkali atom. Here, we have discussed some important trends in physical properties of alkali metals as we go down the column.
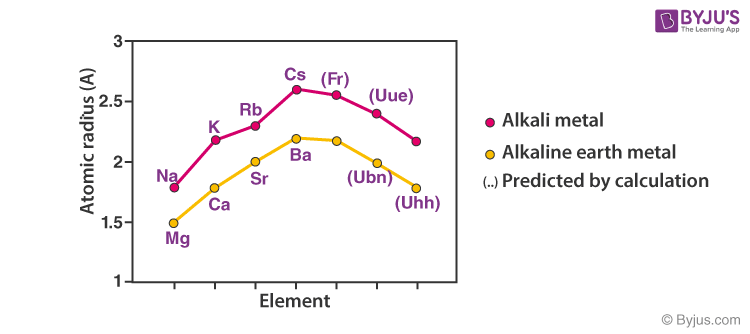
Atomic and Ionic Radii of Elements
Atomic and ionic radii of elements increase, regularly down the column. Also, every alkali metal has the largest radii than any other element in the corresponding period.
Atomic Radii of Alkali Elements
Alkali metals readily lose an electron and become cationic. The cationic radius is smaller than the neutral atom. The relative ionic radii also increase down the column.
Increasing order of Atomic and Ionic Radius: Li ˂ Na ˂ K ˂ Rb ˂ Cs and Li+ ˂ Na+ ˂ K+ ˂ Rb+ ˂ Cs+
Density of Alkali Metals
Having the largest radius and volume, alkali elements have the lowest density. So they are very soft and can be cut with a knife. Lithium, sodium and potassium are lighter than water. Potassium has the lowest density among alkali metals.
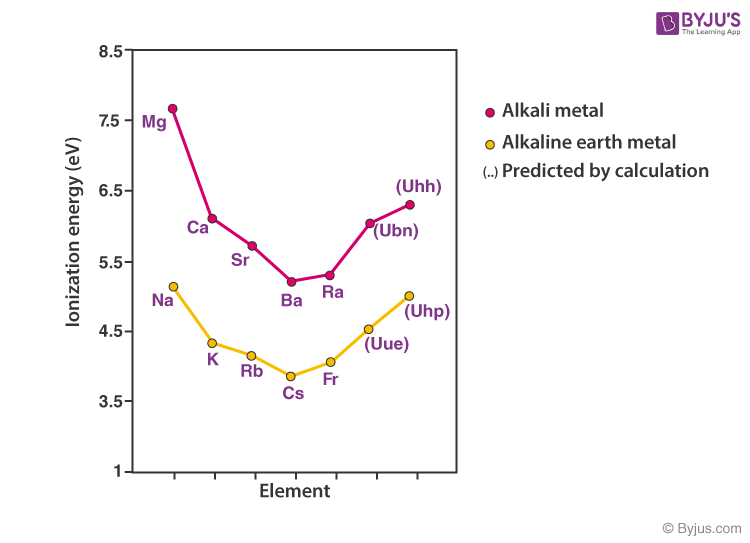
Electropositive Metallic Character and Ionization Energy
Alkali metals shall donate the single valence electron to get a noble gas configuration. Thus they are all univalent electropositive metals. Ionization energy needed for the removal of the valence electron will be highest for the small lithium atom.
With increasing atomic size, the valence electron gets shielded by the inner electrons and becomes easily removable with less energy requirement. Hence the ionization energy decreases with an increasing atomic number.
Ionization Energy – Alkali Metals vs Alkaline Earth Metals
Increasing order of Ionization Energy: Li > Na > K > Rb > Cs
Solubility or Hydration of Alkali Metal Ions
Lithium-ion is the most soluble and the solubility decreases with increasing size so that Cesium ion is the least water-soluble alkali metal ion. Solubility in water is related to the ionic nature and size. Smaller ions have higher charge density and can be solvated by more water molecules. This releases a higher enthalpy of hydration and makes the hydrated ions more stable.
Solubility of Li+ > Solubility of Na+ > Solubility of K+ > Solubility of Rb+ > Solubility of Cs+
Reduction Potential
The substances that can donate electrons are reducing agents. Reducing ability is, related to the ease of electron donation or lower ionization energy.
As ionization energy decreases down the column, reducing property is expected to increase from Lithium to Cesium. While, reducing ability increases from Sodium to Cesium, Lithium has the highest reduction potential (-3.04V) and is the strongest reducing agent of all elements.
Reduction potential and reducing ability depends on the combined energy difference of three processes:
- Sublimation of the atom,
- Ionization to the metal ion,
- Hydration of the ion with water.
Lithium, being the smallest ion, its hydration enthalpy is very high than others and compensates more than its higher ionization enthalpy: ENa ˂ EK ˂ ERb ˂ ECs ˂ RLi
Flame Colouration
In s-block elements, the energy needed for an electronic transition between the available energy levels falls in the visible spectrum region.
So, on heating, they produce a characteristic colour to the flame reflective of their emission or absorption spectrum and can be used for their identification.
Why are Melting and Boiling Points of Alkali Metals Low?
Being very soft, alkali metals have low melting and boiling points compared to the other period elements. Melting and boiling points decreases from Lithium to Cesium.
Chemical Properties of Alkali Metals
These metals are highly electropositive and form compounds which are ionic in nature. Here, we will talk about the different compounds of alkali metals and their general characteristics.
Hydrides:
Alkali metals react with hydrogen at higher temperatures to form metallic hydrides. Metallic hydrides release hydrides ions.
2M + H2 → 2MH → M+ + H–
Nitrides and Phosphides:
Alkali metals can react with even atmospheric nitrogen to form nitrides.
6M + N2 → 2M3N
Phosphorus, form similarly phosphides. Water hydrolyses phosphides to phosphine.
3M + P → M3P →(H2O) 3MOH + PH3
Oxides:
Alkali metals react with atmospheric oxygen and get tarnished of their shining nature. They burn with oxygen to form oxides. But, the nature of oxides formed is different.
Oxygen has a different oxidation state in them. Smaller Lithium forms a normal oxide, while sodium forms peroxides and the larger atoms form superoxides.
- 4 Li + O2 → 2Li2O (Oxide, Oxidation Number of Oxygen= -2)
- 2 Na + O2 → Na2O (Peroxide; Oxidation Number of Oxygen= -1)
- K/Rb/Ce + O2 → (K/Rb/Ce)O2 (Superoxide; Oxidation Number of Oxygen= -1/2)
Since the alkali metals react with nitrogen, oxygen and water in the air, they are always stored under kerosene.
Hydroxides of Alkali Metals:
The reaction of Alkali Metal with Water
Alkali metals react with water to form basic hydroxides and liberate hydrogen. The reaction of the metal is exothermic and the enthalpy increases from lithium to cesium. Alkali metal floats on the water during the reaction.
The density of Sodium and potassium are lower than water. In heavier alkali metal, reaction enthalpy is high such that the metal gets melted and raises to the surface. Hence, the reaction with water becomes faster, highly exothermic, and explosive leading to fire from lithium to cesium.
2M + 2H2O → 2M+ + 2OH– + H2 + ∆H
With the largest electrode potential and high hydration energy, lithium is expected to be more reactive and highly exothermic. Instead, lithium reaction with water is slow and not explosive. Lithium has higher ionization energy and more covalent than rest of the alkali metal ions and so its solubility and the amount reacting will be limited. Cesium is ionic and soluble in water.
Moreover, the enthalpy of reaction is higher than that the latent heat of fusion. So the cesium melts into liquid increasing the amount reacting leading to more reaction as a cycle. Alkali metals can replace hydrogen from any proton donor molecules like alkynes, ammonia, alcohol etc.
Oxides and Water:
Metal and their oxides react with water to ultimately yield hydroxides.
- Li2O + H2O → 2LiOH
- Na2O2 + 2H2O → 2NaOH + H2O2
- 2KO2 + 2H2O → 2KOH + H2O2 + O2
Carbonates and Bicarbonates:
The hydroxides are alkaline which react with carbon dioxide to carbonates.
2NaOH + CO2 → Na2CO3 + H2O
Alkali metal carbonates except lithium carbonate are ionic, thermally stable, and water-soluble. These properties increase from lithium carbonate to carbonate. Bicarbonates, except lithium bicarbonate, are solid, water-soluble and on heating liberate carbon dioxide.
Sulphates:
Sulphates except lithium are soluble in water. Sulphates can be reduced by carbon to sulphide. Forms double salts with trivalent metal sulphates (alum).
Nitrates:
Nitrates are soluble in water and on heating except lithium nitrate decomposes to nitrites.
2MNO3 → 2MNO2 + O2
Halides:
Alkali metals react vigorously with all the halogens to form solid ionic halides with a definite crystal structure. Reactivity decreases from fluorine to iodine. Lithium halides are an exception with more covalent bonding because of the high polarization of the small covalent ion on the electron cloud of the halogen anion as indicated by the Fajan’s rule.
Lithium halides are insoluble in water. Halides of bigger metals form poly halides by combining with more halogens.
KI + I2 → KI3
The Reaction of Alkali Metals with Liquid Ammonia
Alkali metals ionize into cations and electrons in liquid ammonia. The cation and the electrons get solvated by the ammonia molecules. The solution is electrically conductive, reductive, and paramagnetic.
The solvated electrons absorb in the visible region and the solution turns blue in colour. On standing, colour changes into bronze colour and the solution becomes diamagnetic. In dilute solutions, the cation, electron and ammonia react to form sodamide and hydrogen gas.
M + (x + y)NH3 → [M(NH3)x]+ + [M(NH3)y]– → MNH2 + ½H2
Dry ammonia gas reacts with hot metal to form an amide. The amide is hydrolyzed to ammonia.
- 2M + 2NH3 → 2MNH2 + H2
- NaNH2 + H2O → NaOH + NH3
Extraction of Alkali Metals
The usual method of extraction is not applicable to the extraction of alkali metals. Being the highest electropositive metals, displacement by other metals and electrolysis are not applicable. Also, high electrode potential restricts reducing agents like carbon to reduce them.
In electrolysis of aqueous solution, hydrogen ions get preferentially reduced to gaseous hydrogen than sodium ion. Hence, Sodium and potassium are obtained only by the electrolysis of the fused salts of sodium hydroxide and sodium chloride. Alkali metals form alloys with themselves, other metals, and amalgams with mercury.
Anomalous Behaviour of Lithium
Lithium differs from other alkali metal it has more covalent nature due to its smallest size, highest ionization energy, strongest electropositive and polarizing nature. Also, lithium has the strongest reducing character because of its smaller size, larger solubility, and highest electrode potential.
Anomalous Behavior
- It is harder than other alkali metals.
- Reacts slowly with oxygen to form a normal oxide that does not get tarnished quickly.
- Lithium reacts very slowly with water.
- Lithium hydroxide is less basic. Only lithium hydroxide decomposes back into oxide and water.
- Lithium carbonate is less stable due to covalent nature and decomposes into oxide and carbon dioxide.
- It reacts with atmospheric nitrogen to form nitride.
- Lithium nitrate decomposes into nitrogen dioxide, oxygen and oxide, while the other nitrates of alkali metals yield nitrites and oxygen.
- Lithium form imide while other alkalis form amide with liquid ammonia.
- Lithium salts are less soluble compared to other alkali metal salts.
Diagonal Relationship of Lithium with Magnesium
Lithium of alkali metal group resembles more with the magnesium of alkaline earth metal group.
- Lithium and Magnesium are relatively harder metals with higher melting points.
- Both slowly react with water to liberate hydrogen.
- Water hydrolyzes both nitrides to liberate ammonia.
- Both form normal oxides which are less soluble in water.
- Both form carbide which on hydrolysis yields acetylene.
- Bicarbonates of lithium and magnesium are stable only in solution and not in solid form.
Uses of Alkali Metals
Peroxides of Sodium and Potassium:
Controlled oxidation of alkali metals like sodium and potassium with moisture-free oxygen gas at around 300°C gives peroxides.
2M + O2 → M2O2
Peroxides form hydrogen peroxide with cold water and oxygen at higher temperatures.
- M2O2 + 2H2O → H2O2 + 2MOH
- 2M2O2 + 2H2O → O2 + 4MOH
Alkali metal peroxides are used to produce other peroxides, bleaching, preparing perborate and purification of air in small spaces.
Potassium Superoxide
It is prepared by heating potassium with excess oxygen or passing ozone through potassium Hydroxide. It is an orange solid and paramagnetic. Superoxides of alkali metals are a powerful oxidizing agent due to the release of hydrogen peroxide and oxygen in aqueous solution.
2KO2 + 2 H2O → H2O2 + O2 + 2 KOH
Sodium Carbonate – Na2CO3
It is prepared by the Solvay process. Raw materials needed are brine, carbon dioxide and ammonia. Carbon dioxide is obtained by calcining limestone. Ammonia and carbon dioxide react to form ammonium bicarbonate, which is used to precipitate less soluble sodium bicarbonate from the aqueous solution using brine.
On heating, bicarbonate produces sodium carbonate. Calcium oxide on treatment with water gives calcium hydroxide which on treating with the byproduct releases ammonia for reuse.
CaCO3 + 2NaCl → Na2CO3 + CaCl2
Sodium Bicarbonate
Sodium bicarbonate is precipitated out of a concentrated aqueous solution of sodium carbonate by carbon dioxide.
Na2CO3 + H2O + CO2 → 2NaHCO3
The aqueous solution is alkaline. The bicarbonate ion is amphiprotic i.e. both proton donor and acceptor.
Baking soda
Baking soda is a mixture of sodium bicarbonate and weak solid organic acids like tartaric acid and a diluent like cornstarch. The mixture produces carbon dioxide by the reaction between the acid and the carbonate giving a porous structure in baking products.
Hydroxides
Hydroxides are produced by the electrolysis of an aqueous solution of brine. Hydrogen and chlorine are obtained as the by-products. Hydroxides of alkali metals are strong bases. They are deliquescent and form carbonate by reacting with carbon dioxide. Some metal salts of Zn, Al, precipitate metallic hydroxides, which dissolve in excess alkali.
- ZnCl2 + 2 NaOH → Zn(OH)2 + 2NaCl
- Zn(OH)2 + 2 NaOH → Na2 ZnO2 + 2 H2O


